MATTER
Everything in the world is made of matter. Matter is anything that has mass (weight) and occupies space.
Matter can be made up of a group or series of different atoms to form a molecule. These groups of atoms (molecules) are sometimes called compounds. Some types of matter can be broken down to a single atom while still maintaining the properties of the original material. These types of material are called elements.
Matter has three states: Solid, Liquid, and Vapor.

MOLECULE EXAMPLE
Imagine a lake. Now imagine taking the smallest particle or piece of water from the lake. You would have a single molecule of water, H2O, which is made up of two hydrogen atoms and one oxygen atom.
Not all materials are made up of molecules. Copper, for example, is made up of a single copper atom. These are called elements. Each element is a type of matter that has certain individual characteristics.
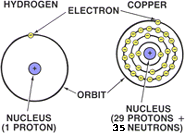
THE ATOM
One of the basic building blocks in the universe for matter is the atom. All matter - gas, liquid, or solid - is made up of molecules or atoms joined together. These atoms are the smallest particle into which an element or substance can be divided without losing its property.
 A single atom consists of three basic components: a proton, a neutron, and an electron. A single atom consists of three basic components: a proton, a neutron, and an electron.
Within the atom there is a Nucleus. The Nucleus contains the protons and neutrons. Orbiting around the nucleus are the electrons.
An atom is similar to a miniature solar system. As with the sun in the center of the universe, the nucleus is in the center of the atom. Protons and Neutrons are contained inside the nucleus. Orbiting around the nucleus are the electrons.
 ATOM CONSTRUCTIONAn atom is similar to a miniature solar system. As the sun is in the center of the solar system, so is the nucleus is in the center of the atom. Protons and neutrons are contained within the nucleus. Electrons orbit around the nucleus, which would be similar to planets orbiting around the sun. ATOM CONSTRUCTIONAn atom is similar to a miniature solar system. As the sun is in the center of the solar system, so is the nucleus is in the center of the atom. Protons and neutrons are contained within the nucleus. Electrons orbit around the nucleus, which would be similar to planets orbiting around the sun.
NUCLEUSThe Nucleus is located in the center of the atom (shown in red).
 The Nucleus contains the protons and neutrons. The Nucleus contains the protons and neutrons.
Orbiting around the nucleus are the electrons.

PROTONS Protons are located within the nucleus of the atom (shown in blue).
Protons are positively (+) charged.
NEUTRONS Neutrons add atomic weight to an atom (shown in green).
Neutrons have no electrical charge.
ELECTRONSElectrons orbit around the nucleus of the atom (shown in yellow).
Electrons are negatively (-) charged.
 Since electrons are lighter than protons and are outside the nucleus, they can be easily moved from atom to atom to form a flow of electrons. Normally electrons are prevented from being pulled into the atom by the forward momentum of their rotation. Electrons are also prevented from flying away because of the magnetic attraction of the protons inside the nucleus, the same type of force that keeps the planets orbiting around the sun. Since electrons are lighter than protons and are outside the nucleus, they can be easily moved from atom to atom to form a flow of electrons. Normally electrons are prevented from being pulled into the atom by the forward momentum of their rotation. Electrons are also prevented from flying away because of the magnetic attraction of the protons inside the nucleus, the same type of force that keeps the planets orbiting around the sun.
ELECTRICAL CHARGES Opposite electrical charges always attract each other. So these particles with opposite charges will tend to move toward each other. Like electrical charges always repel. So particles with like charges will move away from each other.
Remember: Opposites charges attract, and like charges repel.
Atoms always try to remain electrically balanced.
 | |
BALANCED ATOMSAtoms normally have an equal number of electrons and protons.Atoms have no electrical charge. They are neither positive nor negative. They are electrically neutral or BALANCED.The negative charge of the electrons will cancel the positive charge of the protons, thus balancing the charge of the atom.
This cancellation of charges creates a natural attraction or bonding between the positive proton and the negative electron. |
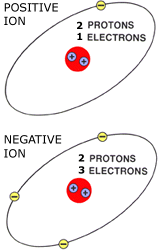 | |
ION PARTICLESWhen an atom loses or gains an electron, an imbalance occurs.
The atom becomes either a positively or negatively charged particle called an ION. These unbalanced charged ION particles are responsible for electron flow (electricity).
IONs will take or release an electron to become balanced again.
|
 | |
ION CHARGEA positive (+) ION has one less electron than it has protons.
A negative (-) ION has one more electron than it has protons.
The positive ION attracts a negative ION to become balanced. This attraction or difference in electrical potential causes electron flow. |
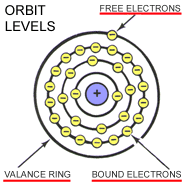 | |
ELECTRON ORBITSElectrons rotate around the atom at different orbits called Rings, Orbits, or Shells.
BOUND ELECTRONS orbit the nucleus on the inner rings. Bound electrons have a strong magnetic attraction to the nucleus.
FREE ELECTRONS orbit on the outermost ring which is known as the VALANCE RING.
|
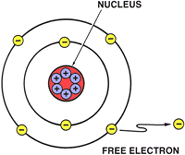 | |
FREE ELECTRONSOnly the FREE ELECTRONS in the outermost shell (Valance Ring) are free to move from atom to atom. This movement is called ELECTRON FLOW.
These FREE ELECTRONS are loosely held and can easily be moved to another atom or ion.
Because of their distance from the nucleus, free electrons have a weak magnetic attraction. Since this attraction is not as strong to the nucleus as the bound electrons on the inner orbits, the electrons move easily from atom to atom.
|
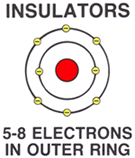 | | INSULATORSAn INSULATOR is any material that inhibits (stops) the flow of electrons (electricity).
An insulator is any material with 5 to 8 free electrons in the outer ring.Because, atoms with 5 to 8 electrons in the outer ring are held (bound) tightly to the atom, they CANNOT be easily moved to another atom nor make room for more electrons.
Insulator material includes glass, rubber, and plastic.
|
|
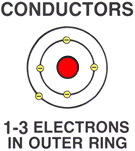 | | CONDUCTORSA CONDUCTOR is any material that easily allows electrons (electricity) to flow.
A CONDUCTOR has 1 to 3 free electrons in the outer ring.Because atoms with 1 to 3 electrons in the outer ring are held (bound) loosely to the atom, they can easily move to another atom or make room for more electrons.
Conductor material includes copper and gold.
|
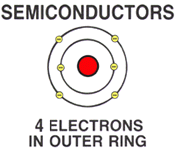 | | SEMICONDUCTORSAny material with exactly 4 free flectrons in the outer orbit are called SEMICONDUCTORS.
A semiconductor is neither a conductor or insulator.
semiconductor material includes carbon, silicon, and germanium.
These materials are be used in the manufacturer of diodes, transistors, and integrated circuit chips.
|
Two Current Flow theories exist. The first is:
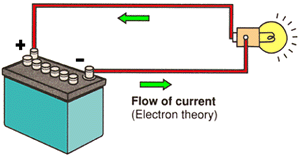
ELECTRON THEORY
The Electron Theory states that current flows from NEGATIVE to POSITIVE. Electrons move from atom to atom as they move through the conductor towards positive. |
|
|
The second Current Flow theory is:
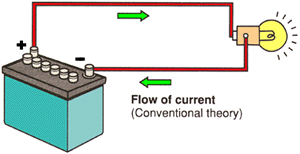
CONVENTIONAL THEORY
Conventional theory, also known as HOLE THEORY, states that current flows from POSITIVE to NEGATIVE. Protons or the lack of electrons (the holes) move towards the negative. (Current flow direction in Hole Theory is the opposite of that in Electron Theory.) |
|
|
VOLTAGE
Voltage is the electrical force that moves electrons through a conductor. Voltage is electrical pressure also known as EMF (Electro Motive Force) that pushes electrons.
The greater the difference in electrical potential push (difference between positive and negative), the greater the voltage force potential. |
|

 | |
MEASUREMENT
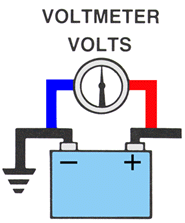
A VOLTMETER measures the voltage potential across or parallel to the circuit.
The Voltmeter measures the amount of electrical pressure difference between two points being measured.
Voltage can exist between two points without electron flow.
|
VOLTAGE UNITSVoltage is measured in units called VOLTS.
Voltage measurements can use different value prefixes such as millivolt, volt, Kilovolt, and Megavolt.
|
VOLTAGE |
LESS THAN
BASE UNIT |
BASIC UNIT |
LARGER THAN
BASE UNIT |
Symbol |
mV |
V |
kV |
Pronounced |
millivolt |
Volt |
Kilovolt |
Multiplier |
0.001 |
1 |
1,000 |
CURRENT (AMPERES)
CURRENT is the quantity or flow rate of electrons moving past a point within one second. Current flow is also known as amperage, or amps for short.
Higher voltage will produce higher current flow, and lower voltage will produce lower current flow.
|

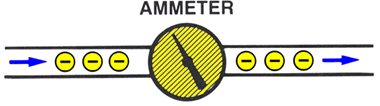
MEASUREMENTAn AMMETER measures the quantity of current flow.Ammeters are placed in series (inline) to count the electrons passing through it.
Example: A water meter counts the gallons of water flowing through it.
|
|
AMPERAGE UNITSCurrent flow is measured in units called Amperes or AMPS.
Amperage measurements can use different value prefixes, such as microamp, milliamp, and Amp.
|
|
AMPERAGE |
LESS THAN
BASE UNIT |
LESS THAN
BASE UNIT |
BASIC UNIT |
Symbol |
µA |
mA |
A |
Pronounced |
Microamp |
milliamp |
Amp |
Multiplier |
0.000001 |
0.001 |
1 |
|
AFFECTS OF CURRENT FLOWTwo common effects of current flow are Heat Generation and Electromagnetism.
HEAT: When current flows, heat will be generated. The higher the current flow the greater the heat generated. An example would be a light bulb. If enough current flows across the filament, it will glow white hot and illuminate to produce light.
ELECTROMAGNETISM: When current flows, a small magnetic field is created. The higher the current flow, the stronger the magnetic field. An example: Electromagnetism principles are used in alternators, ignition systems, and other electronic devices.
RESISTANCEResistance is the force that reduces or stops the flow of electrons. It opposes voltage.
Higher resistance will decrease the flow of electrons and lower resistance will allow more electrons to flow.
|
|
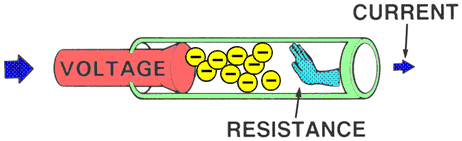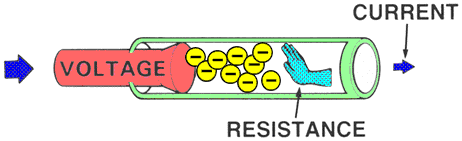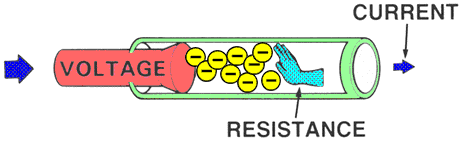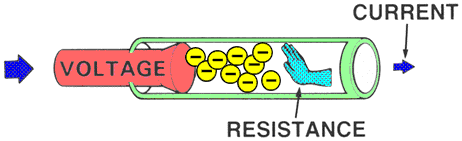
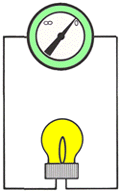 | | MEASUREMENTAn OHMMETER measures the resistance of an electrical circuit or component. No voltage can be applied while the ohmmeter is connected, or damage to the meter will occur.
Example: Water flows through a garden hose, and someone steps on the hose. The greater the pressure placed on the hose, the greater the hose restriction and the less water flows. |
|
|
RESISTANCE UNITSResistance is measured in units called OHMS.
Resistance measurements can use different value prefixes, such as Kilo ohm and Megaohms.
|
AMPERAGE |
BASIC UNIT |
MORE THAN
BASE UNIT |
MORE THAN
BASE UNIT |
Symbol | |
K |
M |
Pronounced |
Ohm |
Kilo ohm |
Megaohm |
Multiplier |
1 |
1,000 |
1,000,000 |
|
RESISTANCE FACTORS
Various factors can affect the resistance. These include:
LENGTH of the conductor. The longer the conductor, the higher the resistance.
DIAMETER of the conductor. The narrower the conductor, the higher the resistance.
TEMPERATURE of the material. Depending on the material, most will increase resistance as temperature increases.
PHYSICAL CONDITION (DAMAGE) to the material. Any damage will increase resistance.
TYPE of MATERIAL used. Various materials have a wide range of resistances.
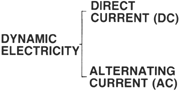
TYPES OF ELECTRICITY
Two basic types of Electricity classifications:
STATIC ELECTRICITY is electricity that is standing still. Voltage potential with NOelectron flow.
DYNAMIC ELECTRICITY is electricity that is in motion. Voltage potential WITH electron flow. Two types of Dynamic electricity exist:
Direct Current (DC) Electron Flow is in only one direction.
Alternating Current (AC) Electron flow alternates and flows in both directions (back and forth).
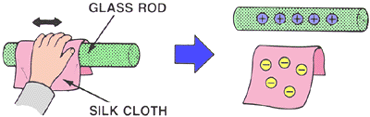
STATIC ELECTRICITY
Voltage potential with NO electron flow.
Example: By rubbing a silk cloth on a glass rod, you physically remove electrons from the glass rod and place them on the cloth. The cloth now has a surplus of electrons (negatively charged), and the rod now has a deficiency of electrons (positively charged).
Another example: Rub your shoes on a rug and then touch a metal table or chair .... Zap!! The shock you felt was the static electricity dissipating through your body. |
|
|
 | | DYNAMIC ELECTRICITY
is electricity in motion, meaning you have electrons flowing, in other words voltage potential WITH electron flow.
Two types of dynamic electricity exists:
Direct Current (DC)
Alternating Current (AC) |
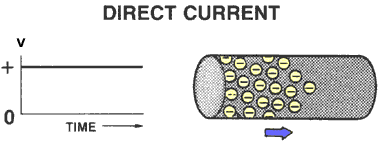
DIRECT CURRENT (DC)
Electricity with electrons flowing in only one direction is called Direct Current or DC.
DC electrical systems are used in cars.
|
|
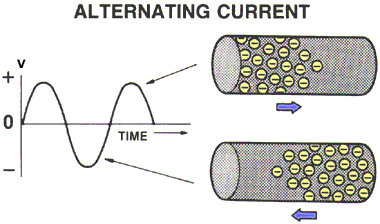
ALTERNATING CURRENT (AC)
Electricity with electrons flowing back and forth, negative - positive- negative, is called Alternating Current, or AC.
The electrical appliances in your home use AC power.
|
|
SOURCES OF ELECTRICITYElectricity can be created by several means: Friction, Heat, Light, Pressure, Chemical Action, or Magnetic Action.
Only a few of these sources of energy are used in the automobile. The battery produces electricity through chemical action, and the alternator produces electricity through magnetic action.
Friction creates static electricity.
Heat can act upon a device called a thermo couple to create DC.
Light applied to photoelectric materials will produce DC electricity.
Pressure applied to a piezoelectric material will produce DC electricity.
Chemical Action of certain chemicals will create electricity. |
|
|
























nhiệt kế điện tử CH-107
ReplyDeletemáy đo nhiệt độ Testo 435-3
máy đo nội trở ắc quy Hioki BT3554
Bút thử điện áp Kyoritsu KT171
Máy đo chất lượng không khí Fluke 975V
máy test cáp mạng Extech CT40
Máy dò bức xạ tia X Huatec FJ-7100
ReplyDeleteMáy đo rung Tenmars ST-141
Máy dò bức xạ Tenmars TM-92
Máy đo điện trở cách điện Kyoritsu 3552BT
máy dò khí Senko
Ampe kìm Fluke 355
Now day, everything is going to find a new but well settled and successful stream for their career. When I came to this blog, I really impressed by all the knowledge points mentioned here. Thank you for this assistance.Tia 568 c
ReplyDelete